Substitution of soybean meal with detoxified Jatropha curcas kernel meal: Effects on performance, nutrient utilization, and meat edibility of growing pigs
Article information
Abstract
Objective
The study was conducted to investigate the effects of replacing soybean meal (SBM) with different levels of detoxified Jatropha curcas kernel meal (DJM) in growing pig diets on growth performance, nutrients digestibility and meat edibility.
Methods
A total of 144 pigs with initial body weight of 20.47±1.44 kg, were randomly allocated to 6 dietary treatments with 6 replications per treatment and 4 pigs per replication for a period of 79 days. Six diets (DJM0, DJM15, DJM30, DJM45, DJM60, and DJM75) were formulated using DJM to replace 0%, 15%, 30%, 45%, 60%, and 75% of SBM. From d 37 to 42, feces and urine were total collected from six barrows in each treatment. At day 79, thirty-six pigs were slaughtered for sampling. The feed intake and weight gain were recorded, while the intestinal morphology, digestive enzyme activities, nutrient digestibility and the content of residual phorbol esters in muscles were determined.
Results
The results showed that increasing the replacement of SBM with DJM decreased the parameters including body weight, average daily gain, average daily feed intake, gain-to-feed ratio, weight and villus heights of duodenum, villus height and villus height/crypt depth of jejunum, digestive enzymes (protease, amylase, lipase, and trypsin) activities, and nutrients digestibility (nitrogen deposition, digestibility of nitrogen, energy digestibility, and total nitrogen utilization) (linear, p<0.05; quadratic, p<0.05) and there was no significant difference among DJM0, DJM15, and DJM30 in all measured indices. The highest diarrhea morbidity was observed in DJM75 (p<0.05). Phorbol esters were not detected in pig muscle tissues.
Conclusion
The DJM was a good protein source for pigs, and could be used to replace SBM up to 30% (diet phorbol esters concentration at 5.5 mg/kg) in growing pig diets with no detrimental impacts on growth performance, nutrient utilization, and meat edibility.
INTRODUCTION
Soybean meal (SBM) is still the most important protein source in the pig industries. Recently, SBM production has been continuously reduced globally, however, the demand for SBM has been increased, thus resulting in an increasing difference between the demand and production. According to statistics of SBM used in China, 70% of SBM was imported from abroad. The increasing shortage of domestic supply for SBM has led to a soaring price of SBM and consequently reduced the profitability in pig production. Besides, SBM competes with human food heavily. Therefore, it is required to look for alternative feed sources to replace SBM [1].
Jatropha curcus is a member of Euphoiaceae family and widely cultivated in many tropical and subtropical regions, such as China, Africa, India, and South East Asia, because of its high oil content [2]. Jatropha curcus kernel meal, containing 48% to 64% crude protein (CP) after oil extraction, is an excellent alternative protein [3]. However, Jatropha curcas seeds were found to be toxic to animals, such as mice [4], rats [5], chickens, [6] and carps [7]. The toxicity was ascribed to the presence of toxins or anti-nutritional factors including trypsin inhibitor, lectin (curcin), saponin, phytate, and phorbol esters. Researchers found that the trypsin inhibitor activity and lectin content in Jatropha curcus kernel meal were higher than those in SBM, and the only difference between non-toxic and toxic varieties of Jatropha curcas was that the toxic varieties had phorbol esters [8]. After heat and chemical treatment, trypsin inhibitor activity and lectin could be removed but not phorbol esters (PEs) [9,10]. So the most important toxin in detoxified Jatropha curcas kernel meal (DJM) was phorbol esters which may affect the edibility. There was a previous report that the DJM could be used in common carp diet to replace partially SBM or fish meal [11]. Our previous study had shown that DJM is a good protein source for growing pigs, and can replace 30% of SBM in the diet [12]. This study was conducted to investigate the effects of DJM in growing pigs on growth performance, enzymes activities, nutrient utilization, and meat edibility, and find out the optimal level of DJM for replacement of SBM.
MATERIALS AND METHODS
Animal care
The present experiment was conducted between September and December 2012 at the Research Farm of Animal Nutrition Institute, Sichuan Agricultural University, Ya’an, China. The experimental protocol used in the present study was approved by the Animal Care and Use Committee of Sichuan Agricultural University.
Animals and treatments
A total of 144 crossbred (Duroc×Landrace×Yorkshire) growing pigs (22.47±1.44 kg) were allotted to six treatments for a 79-day study on the basis of weight, age and sex. The treatments were the diets which replaced 0%, 15%, 30%, 45%, 60%, and 75% of SBM with DJM expressed as DJM0, DJM15, DJM30, DJM45, DJM60, and DJM75, respectively. There were four pigs per pen (2 females and 2 males) and 6 pens per treatment.
Diets and management
The DJM (Table 1) used in this study was obtained from China National Offshore Oil Corporation (CNOOC). The seeds were shelled by husking machine and crushed by rod-toothed crushers bought from Jiangsu Zhengchang Co. Ltd. (Liyang, Jiangsu, China) and pressed into cakes to remove most of the oil which would be reduced to between 14% and 18%. The residual oil and toxins were extracted by solvent-extracted No.6 fuel oil, and then the cakes were detoxified by steam treatment (pre-treatment 80°C and 90°C until dried) and ethyl alcohol extraction (55°C for 2 h) to remove more PEs. The DJM samples were analyzed for moisture, crude fat, CP, crude fiber, ash, calcium, phosphorus [13], amino acid profile [14,15], phorbol esters, trypsin inhibitors activities, and lectin content. Diets were based on corn and SBM and offered to pigs according to a 2-phase feeding programs with live weight from 20 to 50 kg and from 50 to 80 kg. Diets (Tables 2, 3) for each phase were formulated to meet energy and nutrient requirements for growing-finishing pigs as recommended by NRC [16]. Diets were both iso-nitrogenous and iso-calorific for digestible energy among treatments.
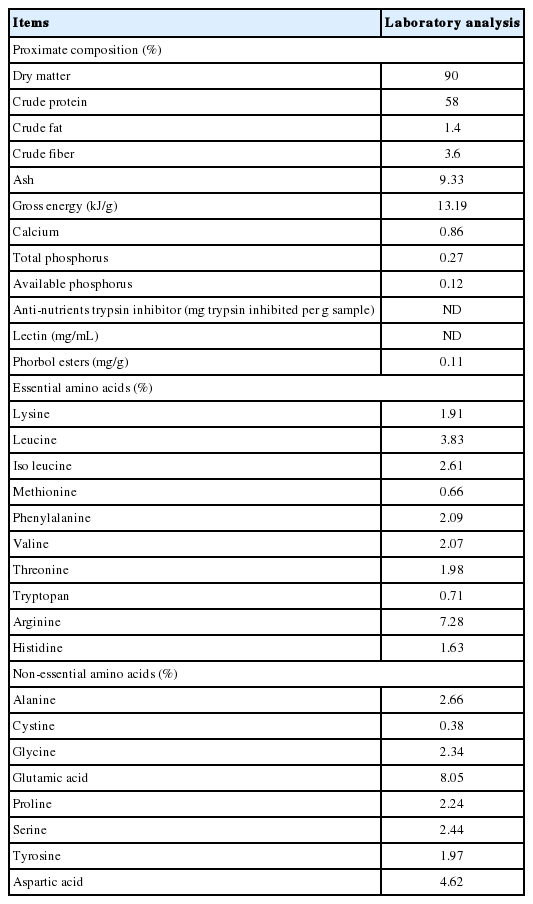
Proximate composition, anti-nutrients content and amino acid profile in Jatropha curcas kernel meal (as fed basis)

Ingredients and chemical composition of the different diets to growing pigs for 20 to 50 kg (as fed basis)
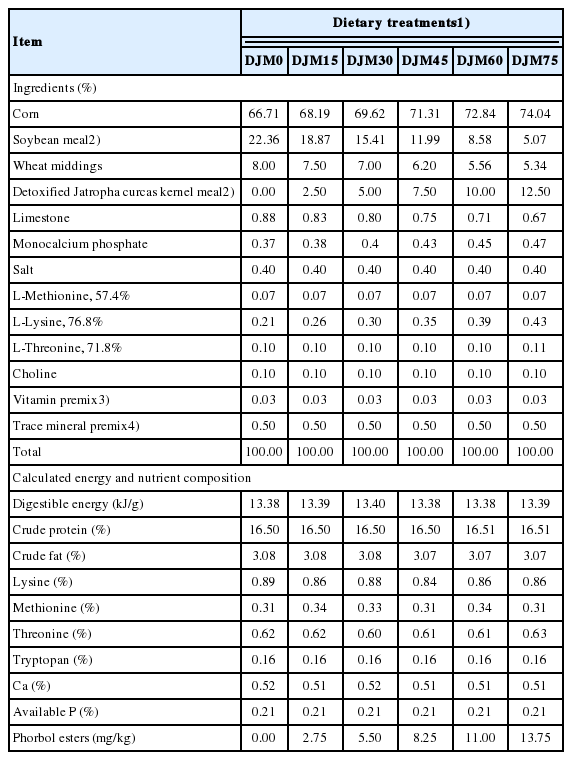
Ingredients and chemical composition of the different diets to growing pigs for 50 to 80 kg (as fed basis)
Pigs were housed in a totally enclosed room with 36 pens (2.0×2.5 m). The room was mechanically ventilated, and temperatures in the room for each phase were 22°C to 28°C and 15°C to 22°C, respectively. Each pen was equipped with a nipple drinking fountain and a feeding hopper (2.0×0.3 m). Water and diets were provided ad libitum throughout the experimental period. Pigs were fed 3 times a day at 08:00, 14:00 and 18:00 h. A sufficient amount of feed was placed in the hopper to ensure that feed was always available. Meanwhile, the hoppers were checked daily to ensure ad libitum access and minimize feed wastage. Daily feed intake per pen was recorded to calculate average daily feed intake (ADFI) and pigs were weighted individually at the end of experiment to calculate average daily gain (ADG). Afterwards, gain-to-feed ratio (G:F) was calculated from ADFI and ADG. Besides, clinical observations (diarrhea and death) of pigs were recorded daily beginning on the first day of the experiment. Feces were assessed visually using the fecal consistency score according to Marquardt et al. [17] with a score from 1 to 3 (1 = well formed, 2 = sloppy, and 3 = diarrhea). The pigs that had diarrhea were identified so that it would not be counted twice. Diarrhea morbidity was calculated as the number of pigs with diarrhea divided by the total number of pigs in the treatment, and mortality was expressed as the number of dead pigs divided by the total number of pigs in the treatment.
At d 29, six borrows per treatment close to the average weight in each group were chosen and moved into metabolism cages for a 14-d experiment, including 8 d for adaption to metabolism cages and 6 d for total collection of feces and urine. Each cage was also equipped with a nipple drinking fountain and a feeding hopper. Water and diets were also obtained ad libitum and daily feed intake per pig was recorded. The diets were same with those in Table 2. Pigs were also fed 3 times a day at 08:00, 14:00, and 18:00 h, and the environmental control was same as before. At d 43, the barrows were moved to their former pens.
Sampling procedure
At the end of 79-d study, a total of 36 pigs, 6 for each treatment close to the average weight in each group, were slaughtered to examine the small intestine. Small intestine was dissected and measured for weight and length quickly after evisceration. The small intestine in growing pigs was defined as the portion of the digestive tract between the pylorus and the ileocecal junction and intestinal segments (duodenum, jejunum, and ileum) were obtained by using the anatomical landmarks, with pyloric-duodenal junction to duodenal-jejunal junction being duodenum, duodenal-jejunal junction to jejunal-ileal junction being jejunum, and jejunal-ileal junction to ileocecal junction being ileum [18]. The relative weight (intestinal weight/body weight) was calculated as the intestinal weight divided by body weight, and the intestinal index (weight/length) was calculated as the intestinal weight divided by the intestine length. Besides, the segments (about 2 cm) of tissues from duodenum, jejunum and ileum were taken for morphological examination, and then immediately rinsed by physiological saline, fixed overnight in 10% neutral formalin for 24 h prepared to make pathological tissue section. The intestinal mucosal of remaining jejunum was scraped off carefully with a glass slide, snap frozen in liquid nitrogen and then stored in −80°C for future analyses. In addition, 50 g sample of muscle tissues (longissimus, psoas major, and semitendinosus) per pig were taken to detect the content of residual PEs.
Mucosal morphology measurements
After the intestinal segments (duodenum, jejunum, and ileum) were fixed in 10% neutral formalin fixative for 24 h, they were treated following the dehydration, clearing and paraffin embedding procedures. Then serial sections of 5 μm thickness were made for histopathological studies, followed by straining with hematoxylin and eosin stain. The samples were examined by microscopy later. Two transverse sections of each duodenum, jejunum, or ileum samples were prepared on one slide for morphometric analysis. A total of 12 to 20 intact, well-oriented crypt-villus units per sample were selected randomly and measured. Villus height was measured from the tip of the villus to the base between individual villus, and crypt depth measurements were taken from the valley between individual villus to the basal membrane. Images of the sections were captured at the magnification of 100× using an Olympus BX51 microscope equipped with a DP70 digital camera (Olympus, Tokyo, Japan). Crypt depth (μm) and villus height (μm) in the small intestine were measured with JD801 morphologic image analysis software, and then villus height/crypt depth (V/C) calculated as the villus height divided by the crypt depth.
Enzyme activity
Activities of brush border enzymes were determined in jejunum mucosa. After thawing, 0.5 g of mucosal scraping was homogenized with ice-cold physiological saline and centrifuged for 15 min at 3,000×g at 4°C. The activities of protease, amylase, lipase, and trypsin were measured by kits according to the instructions of manufacturer (Jiancheng Bioengineering Ltd, Nanjing, China).
Determination of anti-nutritional factors and phorbol esters
Trypsin inhibitors activities in DJM were determined according to Smith et al [19] and Liu and Markakis [20]. Ground DJM samples (0.25 g each) were extracted in 12.5 mL of 0.01 M NaOH at pH 9.4 to 9.6 using an ultra-turrax (20,000 rpm for 5 min) with intermittent cooling. The supernatants were collected after being centrifuged at 3,500×g for 15 min and then centrifuged a second time at 9,500×g. The supernatants were collected and used for the assay after appropriate dilution with distilled water. The results are expressed as mg trypsin inhibited per g of sample.
The lectin content in DJM was analyzed by haemagglutination assay [7]. Ground DJM samples (2 g each) were extracted in 10 mL of phosphate-buffered saline using an ultra-turrax (20,000 rpm for 5 min) with intermittent 5 min cooling using an ice bath. The contents were filtered through No. 540 filter paper and the filtrate was collected. The solution was mixed, and the sedimentation pattern of the erythrocyte suspensions were read after 2 h at room temperature. The haemagglutination activity was defined as the minimum amount of meal in mg per mL of assay medium which produced agglutination.
The residual PEs in the DJM and muscle tissues were detected by high-performance liquid chromatography (HPLC) according to the methods of Makkar et al [7] and Makkar et al [21]. In short, 0.5 g of DJM and dried muscle sample were extracted four times with methanol. An aliquot (20 μL) was loaded on a HPLC reverse-phase C18LiChrospher 100, 5 μm (250×4.6 mm inside dimensions from Merck, Darmstadt, Germany) column. The separation was performed at 22°C and the flow rate was 1.3 mL/min using a gradient elution. The PEs peaks were detected at 280 nm and appeared between 18.0 to 19.5 min. The results were expressed as equivalent to a standard, phorbor-12-myristate 13-acetate. The detection limit of PEs is 3 μg/g samples.
Chemical analyses and calculation
Feces and urine were collected per pig and enclosed in a container with 5% H2SO4 at the bottom daily during 6-d period. Subsequently, the samples of feces and urine were stored at −20°C and 4°C, respectively. At the end of the period, feces were natural thawed, pooled over successive days, and weighted, homogenized, subsampled and heat-dried for chemical analyses. Similarly, urine was pooled, weighted, homogenized and subsampled for chemical analyses. Samples of feces and urine were analyzed for nitrogen content determined by the Kjeldahl method using a Kjeltec 8400 Analyzer Unit (Foss, Sweden) and gross energy with an Parr 1281 isoperibol bomb calorimeter (Parr Instrument Company, Moline, IL, USA). Nitrogen deposition (ND) of each pig was obtained as the difference between nitrogen intake (NI) and nitrogen losses in the feces (NF) and urine (NU). Digestible energy was determined as the difference between gross energy (GE) and fecal energy (FE). The digestibility of nitrogen (DN), total nitrogen utilization (TNU), biological value (BV), and energy digestibility (ED) were calculated as indicated below [22]:
Statistical analyses
The average pen data were used to evaluate effects on growth performance and the pig was considered as the experimental unit for all other variables using the general linear model procedures of SAS (9.0 Inst., Inc., Cary, NC, USA). Variations among the 6 treatments were compared with each other using Duncan’s multiple comparisons. Preplanned single degree of freedom comparisons were also made to measure the linear and quadratic effects of the replacement of SBM by DJM. Values are expressed as means with standard error of the mean. Differences between treatments were considered significant when p<0.05.
RESULTS
Proximate composition, anti-nutrients and amino acid profile in DJM
Proximate composition, anti-nutrients and amino acid profile in DJM are shown in Table 1. The content of CP was 580 g/kg, and the gross energy was 13.19 kJ/g. The content of PEs was 0.11 mg/g, and trypsin inhibitor activity and lectin were not detected in DJM samples.
Growth performance
For all trial periods, increasing replacement of SBM with DJM decreased body weight, ADG, ADFI, and G:F of pigs (linear, p<0.01; quadratic, p<0.01; Table 4) with pigs fed DJM45, DJM60, and DJM75 gaining lower body weight, ADG, ADFI, and G:F than pigs fed DJM0 (p<0.05).
Diarrhea morbidity and mortality
During the experiment period, the highest diarrhea morbidity (Table 4) was observed in DJM75, which was significantly not different from those in DJM30, DJM45, and DJM60 but was higher (p<0.05) than those in DJM0 and DJM15, and only one pig from DJM75 died from serious diarrhea. Diarrhea morbidity demonstrated linear and quadratic effects (p<0.01) in response to increasing replacement of SBM with DJM.
Digestive utilization of dietary protein and energy
Effect of replacing SBM by DJM in the diet on digestive utilization of dietary protein and energy of growing pigs is shown in Table 5. The ND, DN, ED, and TNU of growing pigs were linearly (p<0.05) and quadratically (p<0.05) decreased with increasing replacement of SBM with DJM, but those were not significantly reduced in DJM15 and DJM30 compared with DJM0. No marked difference in BV and digestible energy were observed among all treatments.
Intestinal development
Effect of replacing SBM with DJM in the diets on nutrient utilization of growing pigs is shown in Table 6. Increasing the replacement of SBM with DJM decreased the duodenum weight (linear, p<0.01; quadratic, p = 0.01) and ileum weight (linear, p<0.01; quadratic, p<0.01). The ileum length in DJM15 showed highest among all treatments and significantly higher than that in DJM75 (p = 0.03). The other indexes were not affected by the replacement of SBM with DJM.
Intestinal morphology
The results of morphometric measurements in duodenum, jejunum, and ileum of growing pigs are shown in Table 7. The villus height of duodenum was deceased as the replacement of SBM with DJM increased (linear, p<0.01; quadratic, p = 0.02). The villus heights of jejunum were not affected by the replacement of SBM with DJM. The V/C of jejunum showed a linear decrease with the replacement of SBM with DJM increasing (p = 0.01). Meanwhile, villi of jejunum in DJM75 showed changes such as shorter, disorder and breaking compared with that in DJM0 (Figure 1). No significant decrease was observed in all measured indicates when the replacement was 15% or 30%.
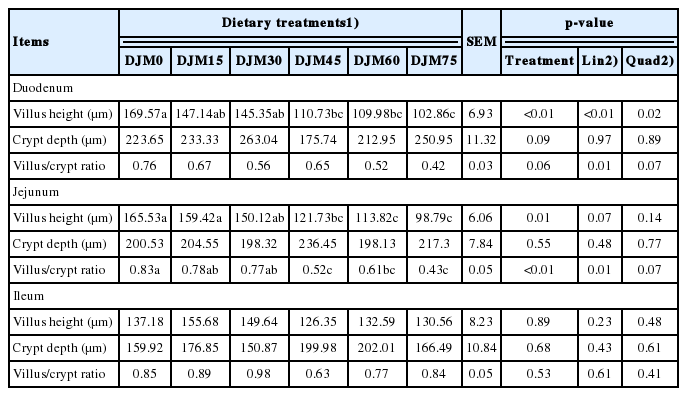
Effect of replacing soybean meal with detoxified Jatropha curcas kernel meal in the diets on morphometric measurements in duodenum, jejunum and ileum of growing pigs

Jejunum morphology of growing pigs. DJM0 and DJM75 were diets in which the detoxified Jatropha curcas kernel meal (DJM) was included in the diets to replace 0% and 75% soybean meal with phorbol esters concentration 0 and 13.75 mg/kg diet, respectively. Intestinal villi of diet DJM75 were shorter compared with that of diet DJM0, and showed changes such as disorder and breaking. 100× magnification.
Enzyme activities
Effect of replacing SBM by DJM in the diets on enzyme activities in jejunum of growing pigs was shown in Table 8. There were linear (p<0.01) and quadratic (p<0.01) effects of different SBM replacements with DJM on the protease, amylase, lipase, and trypsin activities with no significant reduced activities when the replacement was 15% or 30%.
The content of residual phorbol esters in muscles
No PE was detected in the 25 samples of longissimus, psoas major, and semitendinosus (5 samples in each treatment) in DJM15, DJM30, DJM45, DJM60, and DJM75 (Figure 2).
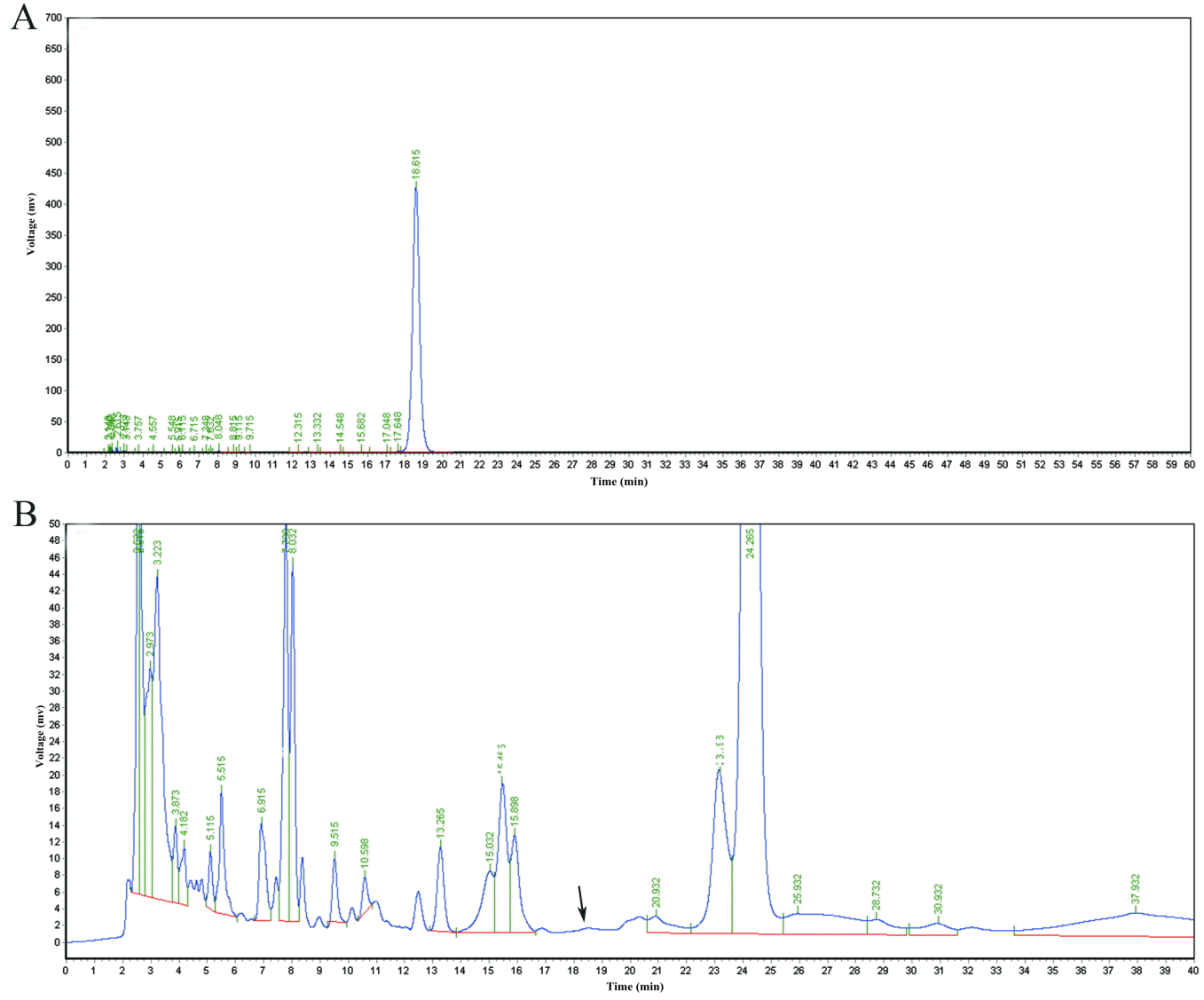
High-performance liquid chromatography profiles of phorbol esters in (A) standardized sample and (B) muscle sample of DJM75. DJM75 was diet in which the detoxified Jatropha curcas kernel meal (DJM) was included in the diets to replace 75% soybean meal with phorbol esters concentration 13.75 mg/kg diet. There was no peak between Time 18 and 20 in muscle sample meaning that no phorbol ester was detected.
DISCUSSION
Many researches showed that DJM was a good protein resource to replace a certain amount of SBM for animals [23]. The crude protein of DJM was higher than SBM, and the levels of essential amino acids except lysine in Jatropha curcas kernel meal were higher than that for the FAO reference protein [10], which were similar with the results from our study. Besides, the pigs fed DJM diets where the replacement was less than 30% showed good ADFI, ADG, and G:F, especially with 15% of replacement, demonstrating the nutritive potential of DJM as a protein source in pig feed. However, when the replacement of SBM by DJM was over than 30%, lower ADFI, ADG, and G:F of pigs were observed. The observed variation in the growth responses might be ascribed to several factors such as palatability, acceptability of diets, presence of toxic and anti-nutritional factors, and digestibility of protein and energy in the diets [24].
Jatropha curcas kernel meal contains many kinds of toxins or anti-nutritional factors [9]. Phorbol esters are considered as the most toxic substance in DJM [24], because they have thermal and chemical stability and can not be completely eliminated [9]. In the present study, there were still some residual PEs (0.11 mg/g) in DJM. The low ADFI might be due to the undesirable characteristics of DJM in terms of taste, smell, texture, and especially the excessive PEs in these diets which had significant negative effects on feed intake [10,25]. The rats fed DJM diets showed significantly reduced feed intake and loss of body weight when the PEs concentration made up to 14.4 mg/kg diet [25]. The DJM could be fed to pigs up to certain levels without detrimental effects on growth performance because pig’s immune system can detoxify low amounts of diet PEs, but it affects growth and development when the rate of PEs deposition exceeded that of metabolism [12,23]. Similarly, Becker and Makkar [26] proved that the threshold level at which PEs appeared to cause adverse effects in carp was 15 mg/kg in the diet. The results of growth performance in the present study suggested that the PEs concentration exceeding 5.50 mg/kg in a diet would decrease the growth performance of growing pigs.
Lower nutrient digestibility and absorption might be the reason for the decreased growth performance. The current study showed that when DJM was used to replace SBM exceeding 30% (diet PEs concentration at 5.50 mg/kg), it decreased the utilization of energy and nitrogen, enzyme activities and damaged intestinal morphology. Small intestine is the main place for absorption of nutrients. Morphology of the intestine is an important marker reflecting the health of the intestine. The villus height determines the ability of small intestine to absorb nutrients, and the V/C is considered as a useful criterion to estimate nutrient absorption capacity of the small intestine [27]. Changes in intestinal morphology such as shorter villi and deeper crypt are associated with the presence of toxins [28]. Chivandi et al [1] observed that all the pigs fed DJM diets in which the PEs were 10.4, 20, 29.6, and 40 mg/kg, respectively showed different degrees of diarrhea and decreased serum α-amylase level of all pigs fed DJM, and the research ascribed those as intestinal injury resulted from the residual PEs in the DJM. Consistently, we found that the intestinal development was restricted and the structure of jejunal villus changed such as disorder and breaking when the replacement of SBM by DJM up to 75% in the present study. Wang [29] also discovered reduced villus height of duodenum and jejunum in chickens fed DJM diets. Similar experimental evidence was also observed in common carp fingerlings and rats. Feeding trials on common carp and rats with excessive PEs concentration in the diets have been reported to cause marked damage in intestinal structure and reduction in digestive absorption of protein and energy utilization [29]. Poor digestion and absorption would seriously affect the animal’s growth and health. The diarrhea in this study might be caused by the interplay of maldigestion and malabsorption of nutrients. Diarrhea was also been found in feeding trials on common carp and rats with Jatropha meal containing PEs [11,30]. The reason the lipase in DJM45 does not decrease may be that a different enzyme has a different tolerance for toxins. Kumar et al [16] also found that the lipase activity would not be affected when the DJM replaced 50% of fish meal protein though amylase and protease activities were decreased. Therefore, the PEs concentration exceeding 5.50 mg/kg in a diet could damage the structure of the small intestine and affect adversely the function of absorption, and then decreased growth performance of growing pigs.
It has been certified that DJM could replace SBM and did not impact the health and production of growing pigs [17]. The previous study showed that no PEs were detected in the livers which are the main metabolic organ of PEs [12]. Accordingly, there was no PEs detected in the muscles by HPLC. The detection limit of PEs is 3 μg/g samples which suggested that the PEs concentration was less than 3 μg/g in the muscles. Makkar and Becker [14,15] reported that the PEs concentration in the edible Jatropha seed was 0.11 mg/g. It indicated that feeding DJM to the pigs would not influence the edibility of meat, which was similar to the result of feeding trial on common carp that no muscle morphology was found in all groups [23].
Above all, DJM in which PEs concentration was 0.11 mg/g could be used to replace SBM for CP supply by maximum 30% (diet PEs concentration at 5.5 mg/kg) in growing pig diet, and does not impact the production of growing pigs and the health of human beings. Besides, an economical and efficient detoxification method should be developed for the application of DJM in the pig production industry to reduce feed cost.
CONCLUSION
The results obtained in the present study indicated that DJM could be used to replace SBM for CP supply up to a maximum 30% (diet PEs concentration at 5.5 mg/kg) in growing pig diet with no effects on growth performance, nutrient utilization, and meat edibility of growing pigs.
Notes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
ACKNOWLEDGMENTS
We are grateful to the Xi’an Oil-Fats Research Design Institute of State Grain Reserve Bureau and CNOOC for providing DJM. This study was supported by the National Public Welfare Research Project (201203015) and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT13083). Please check again!




